By Alexandra KurciskaSeventy years ago, for the first time, humans saw the shape of the molecule containing each of our unique genetic information. The twisted, ladder-like structure of DNA is an image that many of us are familiar with today, but this discovery revolutionized molecular biology and continues to impact the world today. We now live in an age where modifying the very thing that makes you you, your DNA, is not just a goal for the future, but a tool at our disposal. The field of gene therapy has rapidly grown in recent years. With discoveries such as the genome editing system, CRISPR-Cas9, revolutionizing the way we think about medicine and how care can be personalized down to the molecular level.
In June 2023, the US Food and Drug Administration approved the first gene therapy (Elevidys) for patients ages four through five with Duchenne muscular dystrophy (DMD), a rare genetic disorder that affects one in every 3,300 males and leads to weakness and atrophy of the body’s muscles. The cause of this disease is a mutated gene that prevents the creation of the protein called dystrophin, which is vital for the health of muscle cells. Without this protein, individuals with DMD have difficulty walking and running, heart issues due to the stress – put on the cardiac muscle, respiratory problems, and other symptoms associated with muscle weakness. Prior to the approval of this therapy, only the symptoms of DMD were treated but the genetic cause was never addressed.
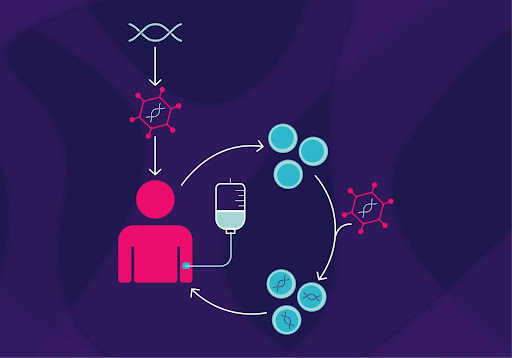
Elevidys is a therapy that is provided via an intravenous dose, delivering a gene into many cells that leads to the synthesis of a protein that can mimic dystrophin, the protein required for normal muscle function. Providing the body with something that it is lacking is one method of gene therapy that introduces the potential for treatments for many diseases which used to be considered untreatable. While a clinical benefit of this medicine is yet to be proven, the FDA is requiring Sarepta Therapeutics, the biotechnology company that created this drug, to conduct a study that observes whether there is an improvement in mobility in DMD patients who have received this therapy. The original FDA approval was granted because of results from a clinical trial that proved that Elevidys did in fact increase the amount of the dystrophin-like protein found in the patients’ bodies.
In fact, because of these results, the FDA approved this drug through the Accelerated Approval pathway, which is reserved only for medicines for extremely serious diseases that do not currently have an effective treatment. The FDA approval of a gene therapy shows that we are on a new horizon of healthcare that is also focusing on diseases that may not be extremely common but are debilitating and, in many cases, life-threatening. Giving patients access to new and potentially beneficial drugs sooner is critical in many cases for genetic disorders when it is a race against the clock as the disease continues to progress. However, patient safety is the most prominent concern, and the FDA must take the clinical risks and benefits into account when coming to this decision.
Another concern that has arisen with the promise of gene therapy is the financial impact of this type of treatment. Elevidys costs $3.2 million per patient, which is the current price tag for a one-time treatment for a rare disease. Questions of how insurance carriers will approach this treatment plan and how the pharmaceutical industry views drugs for rare diseases in terms of profit margins are all valid points that have been brought up as we pass into uncharted territory.
While the approval of Elevidys is a feat of remarkable proportions, we fail to realize we are a long way away from the future of personalized medicine. Scientists continue to uncover more and more about our genetic makeup, but we have many more rungs to climb until we reach the top of the ladder and understand the entirety of the DNA landscape.